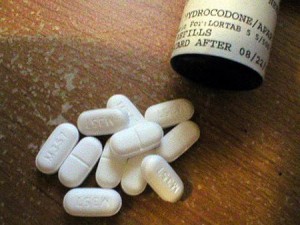
 The U.S. Food and Drug Administration gave its approval this week to a single-ingredient stronger version of hydrocodone, the prescription painkiller that is widely abused.
The U.S. Food and Drug Administration gave its approval this week to a single-ingredient stronger version of hydrocodone, the prescription painkiller that is widely abused.
The regulatory agency said on Friday that it approved Zohydro ER an extended released medication for patients whose pain requires around the clock, daily, long-term treatment, which cannot be treated through the use of other drugs.
Currently hydrocodone is sold in pills that are a combination such as Vicodin that treats pain due to surgery, injury, migraines and arthritis. The new medication from Zogeniz is the first hydrocodone drug that is pure, to be approved for use inside the U.S.
Panel members voted against the medication 11-2, with a single abstention. Members of the panel had questioned the need for another new prescription form of the already widely abused version in the U.S.
Advocates for patient safety quickly criticized the approval of Zohydro, as they had previously urged the U.S. regulatory agency to reject the medication as the public panel had done in December.
One advocate said more children would be killed and then the FDA would say it had made a mistake. The advocate’s son committed suicide in early 2011 while struggling with addiction to painkiller medication.
In 2011, doctors in the U.S. wrote out over 131 million prescriptions for the painkiller hydrocodone, which made it the most prescribed medication n the U.S. Hydrocodone is also consistently ranked amongst the medicines most abused in the United States, according to data from the government.
The drug is part of a family of medications referred to as opioids or opiates because they have a similarity chemically to opium. Others in that group include methadone, codeine, oxycodone, heroin and morphine.
