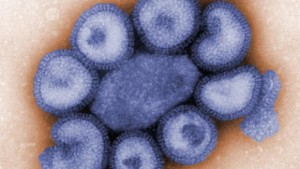
 The U.S. Food and Drug Administration approved a seasonal flu vaccine that is four-strain by pharmaceutical giant GlaxoSmithKline, it was announced Monday. Fluarix Quadrivalent was approved by the FDA to be used for immunization of children three years of age or older and for adults against subtypes A and B of the flu virus.
The U.S. Food and Drug Administration approved a seasonal flu vaccine that is four-strain by pharmaceutical giant GlaxoSmithKline, it was announced Monday. Fluarix Quadrivalent was approved by the FDA to be used for immunization of children three years of age or older and for adults against subtypes A and B of the flu virus.
This is the first intramuscular vaccination that is used to protect against four different flu strains. Vaccines that are three-strain are administered currently to help protect against two common A viruses and one B strain that is predicted to be the predominant strain in that given year, said officials from GlaxoSmthKline.
However, since 2000, there have been two strains of the B virus circulating each season, meaning those patients who are infected with a B virus that is not contained in their vaccine, were not immunized against it.
Glaxo announced the vaccine would start to be available for the start of the 2013-2014 flu season. It plans to also fulfill all orders for its three-strain vaccines. Flu vaccines are typically ordered by healthcare providers one year in advance.
